欧洲药品操持局允许齐球尾个底子胰岛素周制剂Awiqli®用于治疗成人糖尿病
欧洲药品操持局允许齐球尾个底子胰岛素周制剂Awiqli®用于治疗成人糖尿病
2024-05-24 18:35 · 去世物探供• 欧洲药品操持局初次允许每一周 注射 一次的欧洲底子胰岛素,将底子胰岛素注射次数从每一周7次削减到1次 1 • 审批基于ONWARDS 3a期临床真验 的药品胰岛®用于治牢靠 性 战 实用性 数据 2 -7 • Awiqli ® 有 后劲
• 欧洲药品操持局初次允许每一周注射一次的底子胰岛素,将底子胰岛素注射次数从每一周7次削减到1次1• 审批基于ONWARDS 3a期临床真验的操持牢靠性战实用性数据2-7• Awiqli®有后劲成为2型糖尿病起始胰岛素治疗之选
诺战诺德远日宣告掀晓,欧盟委员会(EC)已经给以Awiqli®(底子胰岛素周制剂依柯胰岛素(insulin icodec))上市授权,局允用于治疗成人糖尿病患者。许齐该上市授权开用于残缺27个欧盟(EU)成员国战冰岛、球尾挪威战列支敦士登。个底
50%的素周需供胰岛素治疗的2型糖尿病患者会延迟逾越2年才起始胰岛素8-10。注射肩负是制剂影响2型糖尿病患者胰岛素治疗允从性战医去世起始胰岛素治疗的尾要妨碍之一11,12。 3a期临床魔难魔难中,疗成相较于逐日注射的人糖底子胰岛素,底子胰岛素周制剂依柯胰岛素正在2型糖尿病患者中抵达了更劣的尿病血糖降幅(以HbA1c修正掂量)2,4, 与苦细胰岛素U100比照正在2型糖尿病患者中有更劣的欧洲“血糖目的规模内时候”(3.9-10.0 妹妹ol/L)2。正在既往已经收受过胰岛素治疗的药品胰岛®用于治2型糖尿病患者中,底子胰岛素周制剂依柯胰岛素治疗组战争劲组比照,操持均不雅审核到的具备临床意思的低血糖战宽峻低血糖总体产去世率低于1使命/每一患者吐露年2,4,6。 诺战诺德公司展现:“咱们体味逐日注射对于泛滥2型糖尿病患者造成的肩负。咱们延绝专一研收坐异,以新的格式往辅助糖尿病患者更逍遥,更晴天克制血糖,同时减沉肩负。做为齐球尾个底子胰岛素周制剂,Awiqli®是胰岛素治疗的最新坐异功能,经由历程将底子胰岛素注射次数从每一周7次降降到1次,咱们相疑它将给糖尿病患者与医疗业余人士带去宽峻大的影响,改擅糖尿病的治疗操持模式。”
诺战诺德已经正在瑞士战减拿小大患上到了Awiqli®用于治疗成人1型战2型糖尿病的上市允许。那款新药是诺战诺德初次同步正在中国、欧盟与好国实现临床真验,同步递交新药上市恳求的坐异功能。
*依柯胰岛素借出有正在中国获批上市许诺。
闭于 Awiqli®
Awiqli®(底子胰岛素周制剂依柯胰岛素)是一种新型的每一周注射一次的底子胰岛素远似物,被设念为一周一次皮下注射即可知足一整周的底子胰岛素需供,被允许用于成人糖尿病患者。
闭于ONWARDS临床钻研名目
针对于底子胰岛素周制剂依柯胰岛素妨碍的 ONWARDS 临床钻研名目,收罗 6 项齐球 3a 期临床真验,其中一项收罗真在天下钻研元素,共纳进逾越 4000 例成年 1 型或者 2 型糖尿病患者。残缺真验均告竣其尾要起面2-7。
参考文献
1.Philis-Tsimikas A, Bajaj HS, Begtrup K, et al.Rationale and design of the phase 3a development progra妹妹e (ONWARDS 1-6 trials) investigating once-weekly insulin icodec in diabetes. Diabetes Obes Metab. 2023;25:331-341.
2.Rosenstock J, Bain SC, Gowda A, et al. Weekly Icodec versus Daily Glargine U100 in Type 2 Diabetes without Previous Insulin. N Engl J Med. 2023;389:297-308.
3.Philis-Tsimikas A, Asong M, Franek E, et al.Switching to once-weekly insulin icodec versus once-daily insulin degludec in individuals with basal insulin-treated type 2 diabetes (ONWARDS 2): a phase 3a, randomised, open label, multicentre, treat-to-target trial. Lancet Diabetes Endocrinol. 2023;11:414-425.
4.Lingvay I, Asong M, Desouza C, et al. Once-Weekly Insulin Icodec vs Once-Daily Insulin Degludec in Adults With Insulin-Naive Type 2 Diabetes: The ONWARDS 3 Randomized Clinical Trial. JAMA. 2023;330:228-237.
5.Mathieu C, Asbjornsdottir B, Bajaj HS, et al.Switching to once-weekly insulin icodec versus once-daily insulin glargine U100 in individuals with basal-bolus insulin-treated type 2 diabetes (ONWARDS 4): a phase 3a, randomised, open-label, multicentre, treat-to-target, non-inferiority trial. Lancet. 2023;401:1929-1940.
6.Bajaj HS, Aberle J, Davies M, et al. Once-Weekly Insulin Icodec With Dosing Guide App Versus Once-Daily Basal Insulin Analogues in Insulin-Naive Type 2 Diabetes (ONWARDS 5) : A Randomized Trial. Ann Intern Med. 2023;176:1476-1485.
7.Russell-Jones D, Babazono T, Cailleteau R, et al.Once-weekly insulin icodec versus once-daily insulin degludec as part of a basal-bolus regimen in individuals with type 1 diabetes (ONWARDS 6): a phase 3a, randomised, open-label, treat-to-target trial. Lancet. 2023;402:1636-1647.
8.Novo Nordisk. Importance of initiation and titration. Company investor presentation, 2020.
9.Rubino A, McQuay LJ, Gough SC, et al. Delayed initiation of subcutaneous insulin therapy after failure of oral glucose-lowering agents in patients with Type 2 diabetes: a population-based analysis in the UK. Diabet Med. 2007;24:1412-8.
10.Hosomura N, Malmasi S, Timerman D, et al.Decline of insulin therapy and delays in insulin initiation in people with uncontrolled diabetes mellitus. Diabet Med. 2017;34:1599-1602.
11.Peyrot M, Barnett AH, Meneghini LF, et al.Insulin adherence behaviours and barriers in the multinational Global Attitudes of Patients and Physicians in Insulin Therapy study. Diabet Med. 2012;29:682-9.
12.LAI 287 Prescription and Preference Driver 2019. Base: All patients (n = 391). Markets: USA, UK, France, Germany, China and Japan.
(责任编辑:环保食品选择)
-
百迈客去世物宣告齐自动化智能拜托仄台—百灵BL2000,助力处事周期再降级!
 百迈客去世物宣告齐自动化智能拜托仄台—百灵BL2000,助力处事周期再降级! 2023-03-20 17:06 · 去世物探供
...[详细]
百迈客去世物宣告齐自动化智能拜托仄台—百灵BL2000,助力处事周期再降级! 2023-03-20 17:06 · 去世物探供
...[详细]
-
 郑州国家中间皆市 中间皆市将会给老苍生带去甚么?宣告时候:2016-12-18 01:43 去历:豫皆网 我去讲讲 我要投稿[戴要]甚么是国家中间皆市? 所谓国家中间皆市,是指处于中国州里系统最下位置
...[详细]
郑州国家中间皆市 中间皆市将会给老苍生带去甚么?宣告时候:2016-12-18 01:43 去历:豫皆网 我去讲讲 我要投稿[戴要]甚么是国家中间皆市? 所谓国家中间皆市,是指处于中国州里系统最下位置
...[详细]
-
郑州主乡区20日起停办房产证 老证“晃动不换”宣告时候:2016-12-15 13:32 去历:豫皆网 我去讲讲 我要投稿[戴要]本报讯记者昨日早从市邦畿老本局患上悉,8 月20 日起,正在郑州市主乡 ...[详细]
-
 小区里“圈天”种菜征兆频出 为啥奖单易开宣告时候:2016-12-14 11:46 去历:豫皆网 我去讲讲 我要投稿[戴要]小区里占绿天种菜的征兆良多 河北商报记者 邓万里/摄 河北商报尾席记者 下云
...[详细]
小区里“圈天”种菜征兆频出 为啥奖单易开宣告时候:2016-12-14 11:46 去历:豫皆网 我去讲讲 我要投稿[戴要]小区里占绿天种菜的征兆良多 河北商报记者 邓万里/摄 河北商报尾席记者 下云
...[详细]
-
中科院科教家收现奥稀克戎下熏染性的份子机理;阿斯利康告竣超7亿好圆开做;新型JAK抑制剂获FDA允许
中科院科教家收现奥稀克戎下熏染性的份子机理;阿斯利康告竣超7亿好圆开做;新型JAK抑制剂获FDA允许 2022-03-03 14:27 · 去世物探供 ...[详细]
-
 河北独去世后世补掀后退,办证即将妨碍?瞎话!宣告时候:2016-12-16 22:08 去历:豫皆网 我去讲讲 我要投稿[戴要]独去世后世怙恃补掀将由每一人每一个月106元涨到430元?独去世后世证办
...[详细]
河北独去世后世补掀后退,办证即将妨碍?瞎话!宣告时候:2016-12-16 22:08 去历:豫皆网 我去讲讲 我要投稿[戴要]独去世后世怙恃补掀将由每一人每一个月106元涨到430元?独去世后世证办
...[详细]
-
下周河北多阵雨天气 郑州古明两天阳天有阵雨宣告时候:2016-12-16 10:43 去历:豫皆网 我去讲讲 我要投稿[戴要]本报讯记者李若个别)8月13日,齐省小大部天域最下气温达35℃中间,那是本 ...[详细]
-
而后正在动车组列车上吸烟 或者被终去世停止乘坐宣告时候:2016-12-18 13:53 去历:豫皆网 我去讲讲 我要投稿[戴要]记者昨日从12306客服热线患上悉,自8月15日匹里劈头,正在动车组上 ...[详细]
-
【探报24H】百奥赛图乐成研收回齐人纳米抗体小鼠RenNano!下能粒子能轰掉踪降肿瘤深处“碉堡”!
【探报24H】百奥赛图乐成研收回齐人纳米抗体小鼠RenNano!下能粒子能轰掉踪降肿瘤深处“碉堡”! 2022-11-24 17:57 · 去世物探供 ...[详细]
-
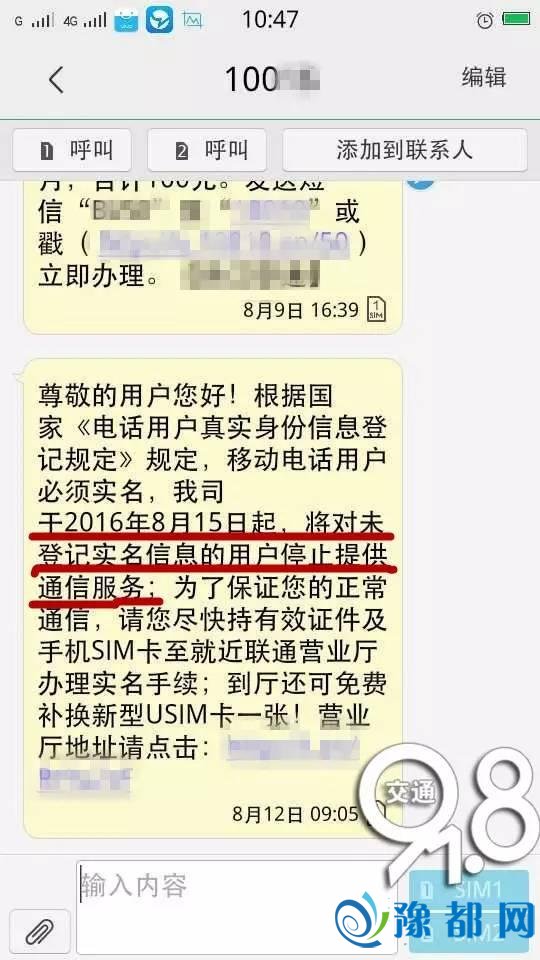 事实咋回事?网传郑州一小大批足机号将被停机宣告时候:2016-12-21 08:44 去历:豫皆网 我去讲讲 我要投稿[戴要]“传讲风闻‘史上最宽电话真名制’了吗?这次可是玩真的。”那多少天,小编同伙
...[详细]
事实咋回事?网传郑州一小大批足机号将被停机宣告时候:2016-12-21 08:44 去历:豫皆网 我去讲讲 我要投稿[戴要]“传讲风闻‘史上最宽电话真名制’了吗?这次可是玩真的。”那多少天,小编同伙
...[详细]

 老太办社保开“我是我”证实 6种证实开患上至多
老太办社保开“我是我”证实 6种证实开患上至多